Aug 21 2025
medical imaging clinical trials
Clinical Research in Medical Imaging: Bridging the Gap Between Data and Real-World Applications
In today’s healthcare landscape, clinical trial imaging has become one of the most powerful tools to transform research data into real-world medical solutions. From drug discovery to medical device clinical trials, imaging technologies now enable researchers to visualize diseases, monitor treatment progress, and validate clinical outcomes.
For researchers, the demand for advanced medical imaging clinical trials is growing rapidly. However, challenges in clinical trial data management, consistency, and quality control often limits the potential of imaging datasets. With services like medical image segmentation, 3D model creation, quality control, and annotation, it is possible to overcome these barriers and accelerate groundbreaking innovations in clinical research.
Why Clinical Trial Imaging Matters
The use of clinical trial imaging is not limited to collecting images only! Rather, it also plays a central role in:
- Accurate study selection through imaging biomarkers.
- Monitoring disease progression across all clinical research trial phases.
- Supporting regulatory approval for drugs and medical devices.
- Reducing variability and clinical errors by standardizing imaging outputs.

When imaging is combined with strong clinical trial data management, it ensures that datasets are not only reliable but also usable in real-world applications.
Challenges in Clinical Research Imaging
Despite its value, clinical trial imaging faces multiple challenges:
1. Clinical Trial Data Management
The volume of images required in clinical research is massive. Without proper clinical trial data management, researchers face storage, accessibility, and compliance issues.
2. Vendor Inconsistency
Different imaging vendors for clinical trials may use varied imaging protocols. This creates discrepancies across multi-site studies.
3. Quality Assurance
High-quality data is critical in medical imaging clinical trials. Any errors in segmentation, annotation, or image quality control can cause biased results.
4. Regulatory Demands
In clinical trials, regulatory agencies like FDA, demand imaging data that is accurate, validated, and reproducible. This requires specialized expertise and strict protocols.
How Our Services Support Clinical Trial Imaging
At Pareidolia Systems LLP, we bridge the gap between data and real-world healthcare applications through specialized imaging services tailored for researchers.
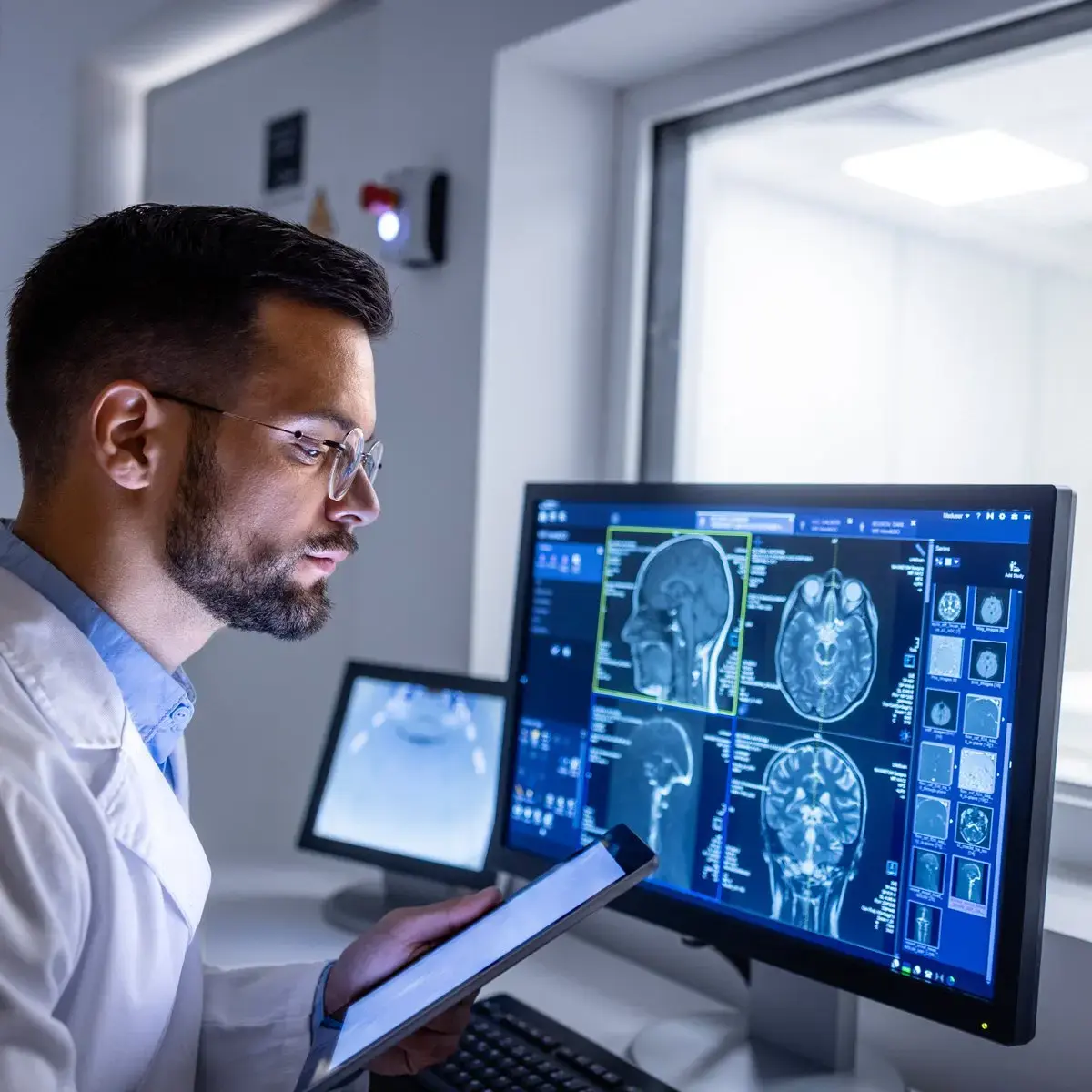
1. Medical Image Segmentation
- Precise segmentation of organs, tumors, or abnormalities.
- Critical for disease tracking in clinical trial imaging.
- Enhances disease tracking, progression analysis, and treatment planning across all clinical research trial phases.
3D Model Creation
- Transforms 2D imaging into realistic 3D anatomical models.
- Widely used in medical device clinical trials for device validation and surgical planning.
3. Quality Control in Medical Imaging
- Ensures consistency and reliability across datasets.
- Strengthens the accuracy of medical imaging clinical trials and regulatory approval.
4. Annotation of Medical Images
- Expert labeling of imaging datasets for AI and ML.
- Essential for both clinical research and the development of future medical solutions.
Clinical Trial Imaging in Real-World Applications
The biggest advantage of clinical trial imaging lies in turning data into practical medical advancements.
- Oncology: Imaging biomarkers are used to track tumor size and therapy response.
- Cardiology: Clinical imaging monitors heart conditions during clinical trials of medical devices.
- Neurology: Medical imaging clinical trials provide insights into brain disorders like Alzheimer’s and Parkinson’s disease.
- Pharmaceutical: Imaging data are used to refine drug discovery and improve clinical trial data management.
With our expert-verified medical image segmentation and quality control services, researchers can ensure their imaging data translates into reliable real-world applications.
Clinical Research Trial Phases & Imaging Integration
Imaging is critical across all clinical research trial phases:
- Phase I: In the early stage of clinical trials, safety assessment is done using imaging biomarkers.
- Phase II: Evaluating drug or device effectiveness with medical imaging clinical trials.
- Phase III: Large-scale validation using standardized imaging datasets.
- Phase IV: Post-marketing studies to monitor real-world outcomes with the help of clinical trial data management.
At every phase, Pareidolia’s expertise ensures that imaging data contributes meaningfully to clinical evidence and regulatory documentation.
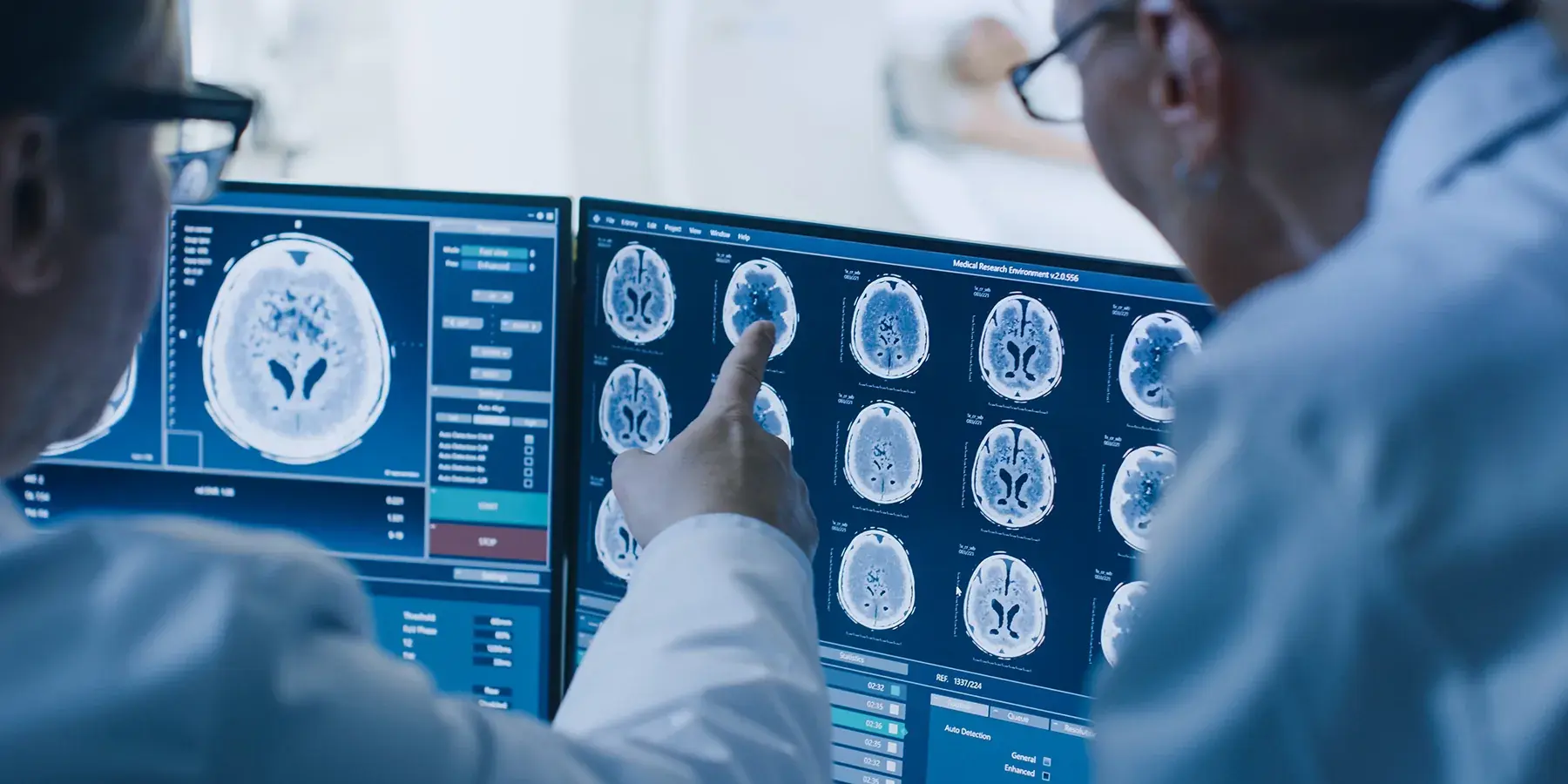
Future of Medical Imaging Clinical Trials
The future of clinical trial imaging is closely tied to AI-driven research and digital health transformation. AI and machine learning, supported by medical image annotation and segmentation, will make trials faster, more accurate, and more cost-effective.
This shift will especially benefit medical device clinical trials and pharmaceutical studies, ensuring that healthcare innovations move from research labs to real-world applications with speed and reliability.
Clinical trial imaging is no longer just a supporting tool—it is the bridge between raw clinical research data and real-world healthcare impact. By combining expert segmentation, 3D modeling, annotation, and quality control, Pareidolia Systems LLP empowers researchers to achieve success in medical imaging clinical trials.
FAQs
Q1: How does data management support imaging in clinical research?
A: Strong clinical trial data management ensures secure storage, standardization, and compliance across global studies.
Q2: What role does Pareidolia play in clinical trials?
A: Pareidolia provides the technical expertise and infrastructure needed to deliver high-quality imaging data so that you can achieve the most accurate outcomes in your clinical trials, every time.
Ready to elevate your Medical Imaging Clinical Trials?
Partner with Pareidolia Systems LLP to ensure your imaging data stands up to the highest standards of quality and compliance. Whether you’re running early-phase trials or seeking regulatory submission, we’re here to help you succeed.
Contact us to discuss your next clinical trials project.